ABOUT US
GET A FREE QUOTE
CONTACT INFO
- +86 19941692089
- info@suntramedical.com
- +86 18133682768
- Location: Room 201-235, Building 21, No. 369, Lushan Road, High-Tech Zone, Suzhou, China.
-
Home
-
News
-
Industry News
- Cybersecurity Becomes Critical Priority for Connected Medical Devices, Driving New Regulatory Standards
Cybersecurity Becomes Critical Priority for Connected Medical Devices, Driving New Regulatory Standards
-
 author
author - 8,December
April 22, 2024 – Medical Device Cybersecurity
As connected medical devices become ubiquitous across hospitals and home care settings, global regulatory bodies—including the FDA and European authorities—are enforcing stricter medical device cybersecurity protocols. Manufacturers are now required to implement end-to-end security measures covering initial design, development, post-market surveillance, and software updates. These regulations aim to safeguard patient data privacy and prevent cyber threats that could disrupt device performance or clinical operations. For healthcare providers, selecting cybersecurity-certified medical equipment is becoming a critical factor in procurement, ensuring both regulatory compliance and operational safety in an increasingly digital healthcare environment.
Table of Contents
Categories
Recent Post
Have Any Questions
-
What are your payment terms / policy?
A: All payments must be made in US dollars unless otherwise specified in the proforma invoice. We offer the following payment options: 1.100% Advance Payment Recommended for standard orders to minimize banking fees. You can process this via Telegraphic Transfer (TT) or Wire Transfer from your local bank to our company account. 2.Partial Advance Payment For larger orders, we require a 30% or 50% down payment. The remaining balance is due before shipment, via Telegraphic Transfer (TT) or Wire Transfer from local bank. 3.Letter of Credit (L/C) Accepted for bulk orders via Sight Letter of Credit. Please note all related bank charges are to be borne by the buyer. 4.PayPal & Credit Card Available for smaller transactions (subject to processing limits). For all bank transfers, please notify us once payment is initiated. Specific currency options and details will be clearly stated in your proforma invoice.
-
What are your main products?
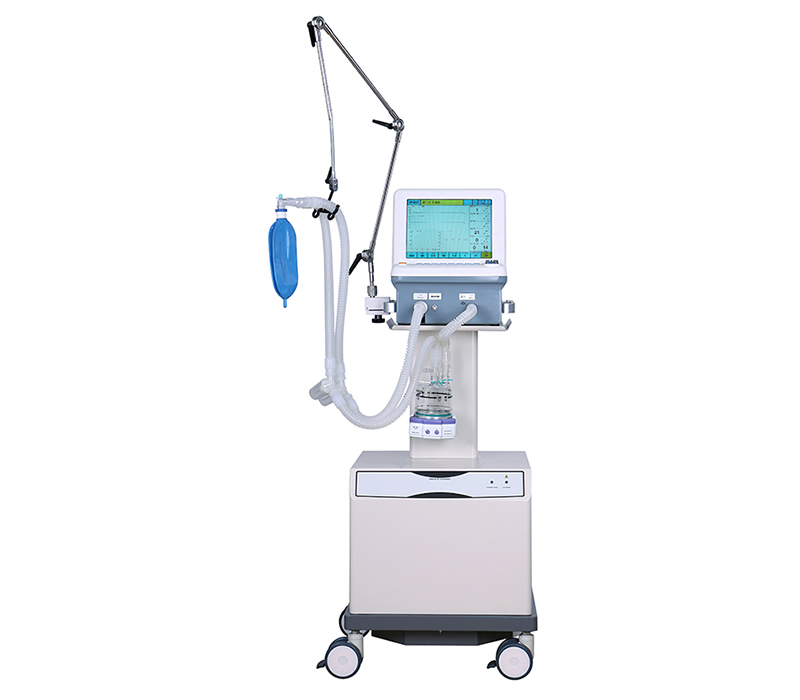
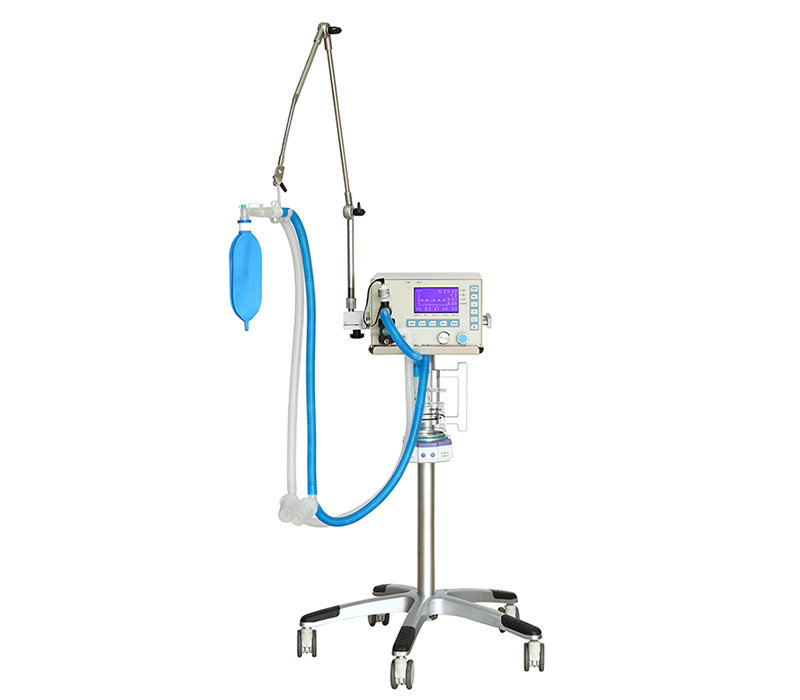
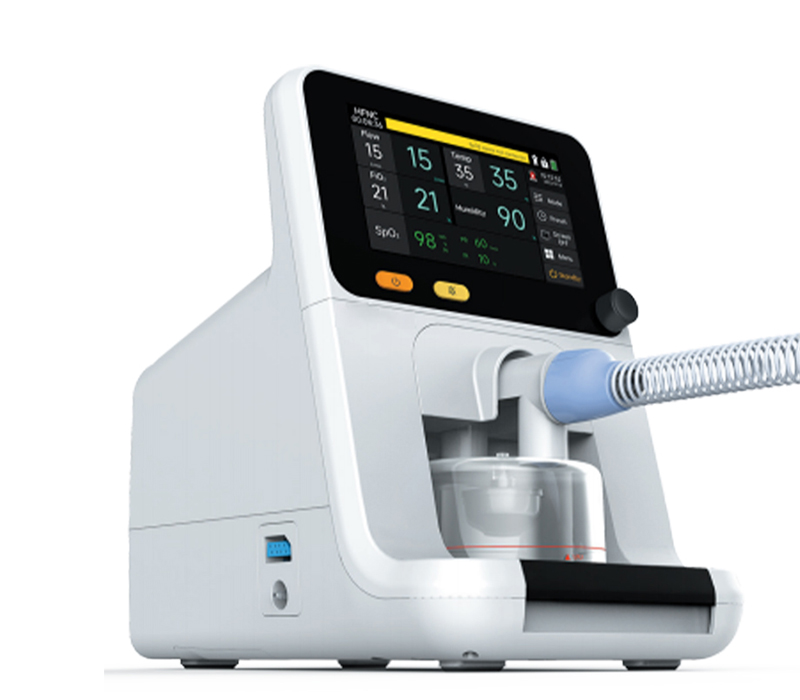
A: SUNTRA provides a comprehensive portfolio of medical equipment spanning multiple specialties. Our product lines include: • Medical Imaging & Radiology • Operating Room & Surgical Solutions • Maternal, Infant & Gynecology Care • Diagnostic & Laboratory Instruments • Sterilization & Infection Control • ENT, Dental & Ophthalmic Equipment • Hemodialysis & Critical Care Systems • Hospital Furniture & Cold Chain Solutions • Veterinary Medical Devices We continuously expand our offerings to meet evolving healthcare needs across these fields.
-
What is your quality assurance and warranty policy?
A: We stand behind the quality of our products with a standard two-year warranty for all medical equipment, effective from the date of shipment. Minor Issues: For minor malfunctions, we will provide free spare parts for replacement. Major Issues: In case of significant failures, we will replace the entire unit free of charge. Important Note: Should the equipment be damaged due to improper handling during transit, the liability rests with the shipping carrier or insurance provider. While this is not covered under our product warranty, our team will actively assist you in filing a claim against the responsible party. Please note that any initial costs for parts or services required during this process will be the customer's responsibility and may be reclaimed through the successful claim.
-
What is your Minimum Order Quantity (MOQ) and delivery time?
A: We maintain a flexible MOQ, starting from just 1 unit for most of our equipment. As our products are made to order, our standard delivery period is approximately 10 working days after order confirmation. For small or sample orders, please inquire about availability, as we may be able to arrange immediate shipment from existing stock.
-
What are the shipping charges and available shipping methods?
A: Shipping costs vary based on shipment size, destination, and transport mode (sea or air freight). For smaller shipments, we typically recommend air freight or express couriers (DHL, UPS, FedEx, EMS, etc.) for faster delivery. For bulk or oversized orders, sea freight is more economical. We work with major carriers such as MAERSK, COSCO, MSC, CMA, and others, and will prioritize your preferred choice while offering suggestions based on schedule and service quality. To provide an accurate quotation, please share your required product quantities, destination port/address, and preferred shipping method.
-
How can I request a quote, price list, or product catalogue?
A: You may contact us by filling out the online inquiry form or sending an email directly to our sales team. To help us provide you with the most accurate pricing and product information, please kindly specify: 1.The product models you are interested in 2.Your detailed requirements or application 3.Any other specifics regarding your inquiry We will send you a comprehensive response with the requested documents within 12 hours.